

Document Type : Original Article
Authors
1 Department of Energy and Applied Chemistry, Usman Danfodiyo University Sokoto, P.M.B. 2346, Sokoto State, Nigeria
2 Department of Chemistry, Sokoto State University, Sokoto, P.M.B 2134, Sokoto State, Nigeria
3 Department of Pure and Applied Chemistry, Kebbi State University of Science and Technology, Aliero. Kebbi State, Nigeria
Abstract
Keywords
Hydrogen is the cleanest fuel known to mankind with no greenhouse gas emissions since the only biproduct of burning hydrogen fuel is water vapor. The applications and uses of hydrogen cannot be overemphasized, as they are many ranging from power production, synthetic fertilizers production, refinery operations, metal processing, food processing, chemicals production, glass production, and electronics industries to mention a few [1-3].
For over 70 years, conventional steam reforming has been the leading technology of hydrogen production. The technology is fully matured and accounts for 48% of global hydrogen production, while other techniques (oil/naphtha, coal gasification, electrolysis, and other processes) represent 52% of hydrogen production combined [4][5].
Global demand for hydrogen is projected to increase in both the chemical and energy sectors. Approximately, 90% of worldwide hydrogen production comes from the use of fossil fuels as feedstock, which arguably contributes to climate change and global warming [4][6-8]. Thus, it is crucial to finetune hydrogen production from sustainable and renewable resources to minimize its environmental footprint.
Since the eighties of the previous century, groundnut production has been rising due to the expansion in its production area and productivity. More than half the production of groundnut in West Africa is produced in Nigeria, which represents 10% of the global groundnut production [9]. Groundnut shell is a leftover product of the groundnut industry. Therefore, and due to the large production quantities of groundnut, using groundnut shells as a feedstock for hydrogen production might be a suitable utilization for this widely available organic residue.
Many researchers have investigated thermodynamic stimulation of hydrogen production using diverse fuel and feedstocks ranging from shale gas [1][2] to methane [11] and including propane [12], hydroxy acetone [13], acetic acid [14], and urea [15] (Table 1). The thermodynamic analysis gives more insight into a system’s overall performance and this undoubtedly leads to finding the sources of losses owing to irreversibility in each process in a system. Furthermore, these simulations play a vital role in making economic and useful process modifications or operational changes [16]. Thus, the main aim of this research was to investigate the theoretical thermal efficiency of conventional steam reforming of groundnut shell.
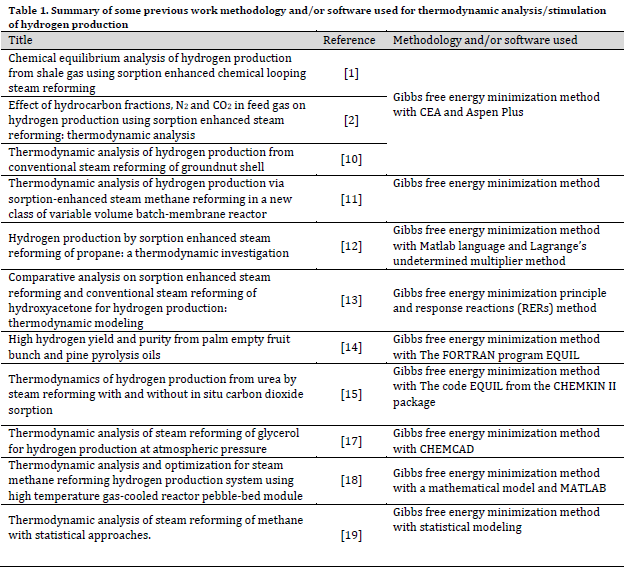
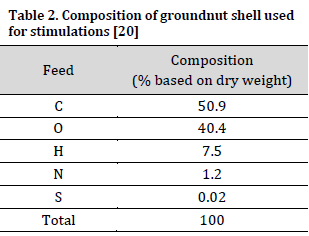
The elemental composition of the groundnut shell feedstock was obtained from the literature [20] (Table 2). The feedstock is readily available in Nigeria as agricultural waste and is among the few biomasses with high hydrogen composition [10][20].
Based on the mentioned feedstock composition, stimulation conditions were arranged with eight steam-to-carbon ratios (S:C) (1 to 8), temperature (700 K to 1200 K), and pressure (1 bar). where ‘C’ represents moles of carbon in the feed, and ‘S’ the moles of water feed as a liquid at 298 K (25℃) that will transform to steam at reforming temperature
Thermodynamic equilibrium calculations of conventional steam reforming of groundnut shell (CSR-GR) were performed using Chemical Equilibrium Applications (CEA) software developed by NASA [21]. The software uses a solution method based on the minimization of Gibbs energy function of a feed mixture to determine the mole fractions of the equilibrium mixture of products.
The thermal efficiency of the process was evaluated using the DH ratio factor. ‘DH ratio’ is the enthalpy of generating 1 mol of hydrogen via the considered equilibrium process divided by that of generating 1 mol of hydrogen via thermal water splitting from reactants in their natural state at 298 K (25℃) and ending with products at reaction temperature. DH ratio can also be described as the measure of energy expenditure of producing hydrogenthrough the groundnut shell-water system compared to the energy gained by the evolution of heat from combusting this hydrogen with oxygen, signifying its final use in a fuel cell or combustion process [1][15]. A DH ratio greater than one (>1) denotes an inefficient process from an energy perspective, since the energy needed to generate hydrogen is greater than the energy released through hydrogen oxidation or combustion in a heat or power-generating device. On the other hand, a DH ratio of less than one (<1) denotes an efficient and theoretically economical hydrogen-generating process from an energy viewpoint. Enthalpy calculations were made based on the following equations [1][2].

Where ∆H is the change in enthalpy, H is the enthalpy of relevant species formation at the indicated temperature, GS stands for groundnut shell, TR reaction temperature, and WSP for water splitting.
DH ratio value was higher than 1 for all the investigated steam-to-carbon ratios except for S:C ratio of 1, where the DH ratio was 1.0 and 1.4 at the temperature of 700 and 750 K respectively (Fig. 1 A). The DH ratio value of 1.0 at 700 K is almost more thermodynamically advantageous to water splitting. The noticed DH ratio increases with temperature increase is expected, since a higher S:C ratio means that more energy is required to heat the excess steam [1][2][15]. Another disadvantage of operating at a high S:C ratio is that a higher reactor volume will be required with increased catalyst deactivation incidents due to pore blockage [1][22][23].
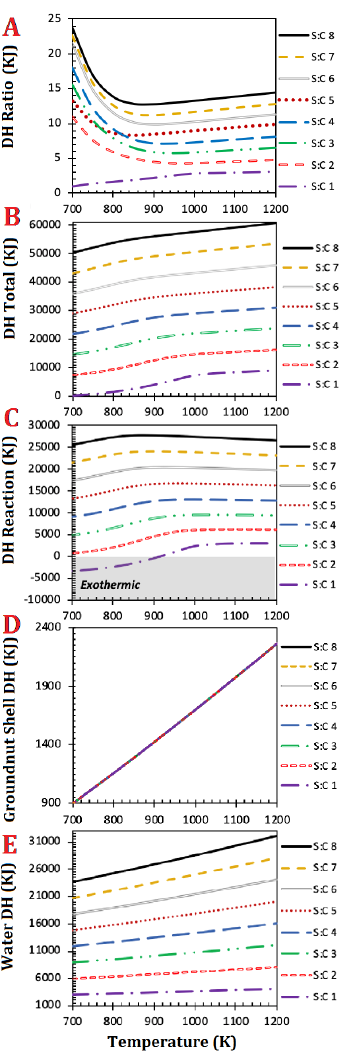
Both the total DH (fig. 1 B) and reaction DH (fig. 1 C) increased with the increase of steam-to-carbon ratio. The total DH at S:C ratio of 1 and 950 K was 5950 kJ while that of 2 at similar operating conditions and temperature was 14143 kJ. This is equivalent to 58 % rise in energy demand between steam-to-carbon ratio of 1 and 2 at exactly same operating conditions. At the temperature of 900 K, which corresponds to the temperature of maximum hydrogen yield for both steam-to-carbon ratio of 7 and 8 [20], the reaction DH at steam-to-carbon ratio of 7 and 8 were 24146 and 27863 kJ respectively, which is equivalent to 13 % increase. The behaviour of the total and reaction DH in the present study is similar to that previously reported in [1][2][15].
The heating demand of the groundnut shell was the same for all the investigated steam-to-carbon ratios as their molar input remained unchanged (Fig. 1 D). On the other hand, the heating demand of water increased with the increase in S:C ratio (Fig. 1 E). Overall, the heating demand of conventional steam reforming of groundnut shell is dominated by the enthalpy of water. The observed unchanged heating demand of feedstock and increased energy demand with the increase in steam-to-carbon ratio is in agreement with previous studies conducted on different feedstocks [1][2][15].
This study shows that the conventional steam reforming of groundnut shell has poor thermal efficiency under the investigated conditions. The energy demand of conventional steam reforming of groundnut shell is dominated by the enthalpy of heating liquid water from 298 K (25℃) to reforming temperature. Thus, energy demand increase with the increase in steam-to-carbon ratio. Therefore, more studies should be conducted to investigate other advanced reforming processes of groundnut shell such as sorption-enhanced steam reforming, chemical looping steam reforming, and sorption-enhanced chemical looping steam reforming and to assess the potential of this feedstock in hydrogen production.
Conflict of interest statement
The authors declared no conflict of interest.
Funding statement
The authors declared that no funding was received in relation to this manuscript.
Data availability statement
The authors declared that all related data are included in the article.