

Document Type : Original Article
Authors
Department of Horticulture and Landscape Design, College of Agriculture, University of Basrah, Basrah, Iraq
Abstract
Keywords
Barberton daisy (Gerbera jamesonii Bolus) is a perennial herbaceous plant that belongs to the Gerbera genus and Asteraceae family [1]. This genus includes about 29-37 species, spread between Africa and Asia [2]. Barberton daisy is considered an important flowering plant used in garden architecture and design [3], and it is ranked fourth as a picking flower in the world after Rose, Chrysanthemum, and Tulip [4]. Its flowers are distinguished by their long life after picking and their resistance to transport damage, in addition to their high prices [5]. The gerbera is characterized by its large flowers with various colors and the floral stalk thickness and hardness [6].
Gerbera plant is commonly propagated by division or suckers, and this method produces a relatively low number of new plants. Furthermore, it is not preferable to use the seed propagation method since the resulting plants are heterozygous and not similar to the mother plant [7]. Researchers resorted to the micro-propagation of gerbera plants for mass production of commercial varieties with high specifications to obtain true-to-type and pathogens free plants using the leaf segment, petiole, shoot tip, and flower bud as explants for in vitro culture [6][8][9] [10].
Aswath et al. [9] were able to regenerate shoots indirectly from the callus derived from cultured gerbera leaf segments on MS (Murashige and Skoog) medium supplied with 2.0 mgL-1 naphthalene acetic acid (NAA) and 1.0 mgL-1 benzyl adenine (BA) with a response to callus induction of 83.3%. Hussein et al. [11] succeeded in obtaining callus from leaf explants by in vitro culture on MS medium with various plant growth regulators (Benzyl adenine, Kinetin, Zeatin, Naphthalene acetic acid, Indole acetic acid, and Indole butyric acid) at different concentrations. The indirect shoots were proliferated from callus cultured on the MS medium supplied with 2.0 mg L-1 NAA and 2.0 or 4.0 mg L-1 BA. The highest numbers of adventitious shoots were obtained using MS medium supplemented with 0.25 mg L-1 IBA and 2.0 mg L-1 BA. This study was conducted to test the effects of various BA and NAA concentrations in culture medium on the micro-propagation aspects of gerbera plants using the young leaves as explants.
This study was conducted at the laboratory of plant tissue culture, College of Agriculture, University of Basrah, Iraq. In this study, young leaves of the two cultivars of Gerbera jamesonii ‘Double Dutch’ and ‘Malibu’ were obtained from a nursery in Baghdad province (Fig. 1 A, B, and C) and used as explants.

The plants were transferred to the laboratory and placed under running water for 30 min to get rid of dust and suspended matter. Then, the young leaves were separated and isolated by a blade (Fig. 1 C) washed with liquid soap and tap water, and then washed thrice with sterile distilled water. The young leaves from each cultivar were then submerged separately in an antioxidant solution of 150 mgL-1 citric acid and 100 mgL-1 ascorbic acid for 30 minutes. After that, the surface sterilization of the explants was carried by immersing the leaves in 70% v/v ethyl alcohol for two minutes and washing them thrice with sterile distilled water. The leaves were then immersed in the fungicide Elsa 50 W (50% carbendazim) at a concentration of 500 mg L-1 while stirring continuously for five minutes, then washed again with distilled water three times. Then, the leaves were placed in a 0.1% w/v mercury chloride solution with 2-3 drops of Tween-20 for 8 minutes and washed with sterile distilled water three times [12].
The culture medium was prepared using 4.44 gL-1 of ready MS medium powder [13] (American Cassion Labs Company). 80 mgL-1 adenine sulfate, 200 mgL-1 sodium hydrogen orthophosphate, 100 mgL-1 myo-inositol, 1.0 gL-1 polyvinyl pyrrolidone, 0.7% w/v agar and 3% w/v sucrose were added to the MS medium. For callus induction and indirect organogenesis stages, different concentrations of BA (1.0, 2.0, 3.0, 4.0, and 5.0 mgL-1), 0.1 mgL-1NAA and 0.5 mgL-1GA3 were added. For the rooting stage, MS medium with different concentrations of NAA (1.0, 1.5, 2.0 and 2.5 mgL-1) and a constant concentration BA (0.1 mgL-1) was used. Then, the pH was adjusted to 5.7- 5.8 with 0.1 N NaOH or HCl. The MS medium was poured at 20 ml into Pyrex culture tubes (2.5 x 18 cm3). Then the nozzles were blocked with medical cotton and aluminum foil, autoclaved under 1.04 kPa at 121 °C for 20 minutes. After culturing, the cultures were incubated in the chamber at 25 ± 2 ºC with 1000 lux light intensity and 16/8 hours of light/darkness cycles. Subculture procedure was carried out every four weeks. Data were recorded for the following traits after three months of culturing:
Experiments were designed according to Randomized Complete Design. Each treatment in these experiments was repeated ten times. The data were analyzed using IBM SPSS Statistics V24.0.0 software. The means of the treatments were compared according to the Revised Least Significant Difference Test at a probability level of 1% [14].
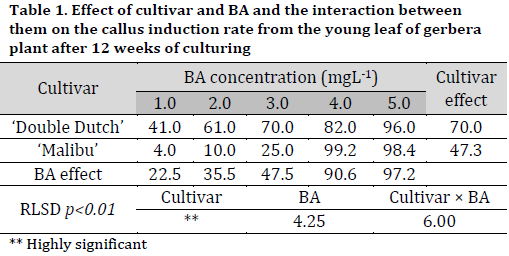
Regardless of BA concentration in MS medium, there were significant differences between cultivars in terms of response to callus induction after 12 weeks of culturing, with ‘Double Dutch’ cultivar having the highest response rate (70%) compared to ‘Malibu’ cultivar (47.32%). Furthermore, significant differences were observed under the different concentrations of BA in MS medium in terms of callus induction rate. MS medium containing 5.0 mg L-1 BA recorded the highest callus induction rate reaching 97.2% (Fig. 1 D), while the MS medium with 1.0 mgL-1 BA recorded the lowest callus induction rate (22.5%). The interaction between cultivar and BA concentration was observed, where the MS medium with 4.0 mg L-1 recording the best induction rate in ‘Malibu’ (99.2%) and 5.0 mg L-1 resulting in the highest callus induction rate in ‘Double Dutch’ callus induction rate (98.4%) (Table 1).
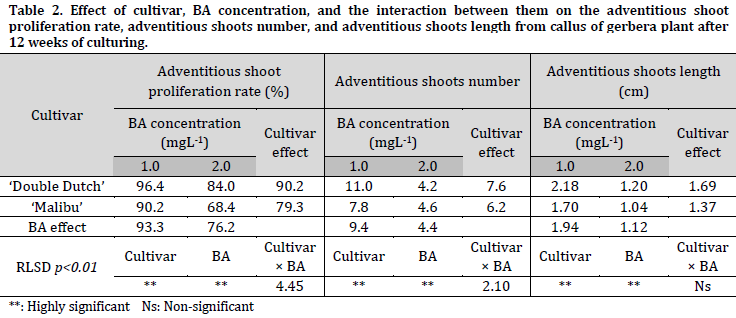
The MS media supplied with 3.0, 4.0, and 5.0 mgL-1 BA did not lead to the regeneration of shoots from induced callus of young leaves (Fig. 1 E and F). The results of this study indicate that there were significant differences between the two studied cultivars (‘Double Dutch’ and ‘Malibu’) in terms of the indirect shoot proliferation rate (90.2% and 79.3%), the shoots number (7.6 and 6.2 shoots/50 mg callus), and the shoots length (1.69 and 1.37 cm), respectively (Table 2). The MS medium supplied with 1.0 mgL-1 BA was the best among the tested BA concentrations, and recorded the highest shoot proliferation, shoots number, and shoots length with an average of 93.3%, 9.4 shoots/50 mg callus and 1.94 cm, respectively (Fig 1, E and F) (Table 2). Additionally, it was observed that the MS medium containing 1.0 mgL-1 BA gave better results for all the studied traits for both cultivars when compared to the medium with 2.0 mgL-1 (Table 2).
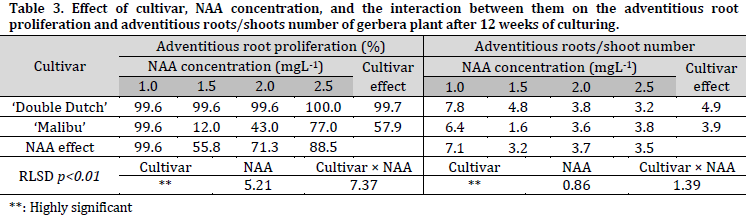
The percentages of rooting response, and the number of adventitious roots, were 99.7% and 4.9 roots/shoot for ‘Double Dutch’, while recorded 57.9% and 3.85 roots/shoot for ‘Malibu’ (Table 3) (Fig. 1 G and H). Furthermore, significant differences between the used NAA concentrations were observed, as the MS medium supplied with 1.0 mgL-1 NAA had the highest rooting response rate and roots/shoot number with 99.6% and 7.1 roots/shoot, respectively (Fig. 1 G and H). The ‘Double Dutch’ cultivar recorded the highest rooting percentage on MS medium supplemented with 2.5 mgL-1 NAA (100%), while the highest roots number was observed with 1.0 mgL-1 NAA (7.8 roots/shoot). The highest rooting percentage and roots number of ‘Malibu’ cultivar were recorded on MS medium supplemented with 1.0 mgL-1 NAA (99.6%, and 6.4 roots/shoot, respectively).
The significant superiority of ‘Double Dutch’ cultivar in terms of callus induction rate and indirect shoot and root proliferation, shoots and roots number, and shoot length compared to ‘Malibu’ cultivar shows the importance of the genetic effects on the studied trait. BA has an important role in stimulating cell division and dedifferentiation. Additionally, the multiple double bonds in BA side-chain increase its effectiveness compared to other cytokinins, leading to the callus induction [15]. The concentration of 5.0 mgL-1 BA may be supra-optimal for stimulating cell division and callus formation compared to its lower concentrations due to its increased effectiveness as it interferes with endogenous hormones in the explants [16]. The current study achieved a callus induction of 97.2% on the MS medium supplied with 5.0 mgL-1 BA, which is higher than the previously reported results obtained by Hossain [17], who showed that the best callus induction was 70% when leaves were cultured on MS medium supplied with 2.0 mgL-1 BA,
The significant superiority of NAA at a concentration of 1.0 mgL-1 in terms of shoots and roots formation is attributed to it being one of the essential synthetic auxins in the initiation and differentiation of roots [18]. The low concentrations of auxins, including NAA, lead to the initiation and formation of adventitious roots on shoots bases when cultured on MS medium in vitro [19]. The high root formation on the MS medium supplied with 1.0 mgL-1 NAA is due to it being the supra-optimal concentration that interferes with endogenous hormones in the shoot tissues that lead to a better initiation and differentiation of roots compared to 1.5, 2.0 and 2.5 mgL-1 concentrations of NAA. The results of this study differ from the results of other studies on other cultivars of the gerbera plant. Previously, it was reported that the concentration of 2.0 mgL-1 NAA in MS medium intended for rooting recorded the highest percentage of response to rooting [10][20].
It can be concluded that the Double Dutch cultivar recorded the highest response to micro-propagation compared to the Malibu cultivar. The BA in high concentrations led to callus induction, and in the low concentrations led to indirect shoots formation. The low concentrations of NAA lead to better rooting response.
Conflict of interest statement
The authors declared no conflict of interest.
Funding statement
The authors declared that no funding was received in relation to this manuscript.
Data availability statement
The authors declared that all related data are included in the article.